Acids, Bases, and Salts are the crucial elements of chemistry, and they take part in lab reactions as well as in daily life. The sourness of lemon juice, for example, is one of the ways these substances are around us, among many others. This guide will lead you through the properties of acids, bases, and salts, the pH scale assessment of their strength, and the creation of new compounds through neutralisation reactions.
This resource, created for IGCSE and Class 10 students, uses simple but thorough concepts illustrated with very clear explanations and real-life examples. Whether you are revising MCQs, working on practice papers, or preparing for past papers focused on acids, bases, and salts, this comprehensive blog by Mixt Academy is sure to improve your understanding as well as your self-confidence.
First of all, acids, bases, and salts are the main classes of compounds in chemistry, considered to be little more than their specific properties as defined by the way they react with each other. Here, you will find a more detailed classification of each of these compounds.

Acids are a category of chemical compounds that, when dissolved in water, produce hydrogen ions (H⁺) as the primary species. They usually have a sour taste, might corrode or be sticky, and change the colour of blue litmus paper to red. Acids are characterised by their low pH (less than 7), and such acids are called acid-base reactions.
The most common examples of acids are ascorbic acid (present in oranges) and hydrochloric acid (found in stomach acid). Acids may be strong (like hydrochloric acid) or weak (like lactic acid in milk), depending on the acid’s character.
Mixt Academy tutors break these concepts into simple, exam-friendly steps—so you learn faster and remember longer.

A base is a substance that releases hydroxide ions (OH⁻)(OH⁻)(OH⁻) when dissolved in water or accepts hydrogen ions (H⁺)(H⁺)(H⁺) during a chemical reaction. Bases typically have a bitter taste, feel slippery or soapy to the touch, and have a pH value greater than 7.
They turn red litmus paper blue and react with acids in neutralisation reactions to form salt and water. Common examples of bases include sodium hydroxide (NaOH) and ammonia (NH₃). Bases are classified as strong or weak depending on how completely they dissociate in water.
Reactivity: Reacts with acids to form salt and water (neutralisation)
Examples: Copper(II) oxide (CuO), Iron(III) oxide (Fe₂O₃)

Salts are a broad group of ionic substances produced when an acid reacts with a base in a chemical neutralisation process. They are made up of positively charged ions (cations) and negatively charged ions (anions) that attract each other through strong ionic forces, forming a compound with no overall charge.
Although many people think only of table salt, salts include a wide variety of substances used in food, medicine, agriculture, and industry. Most salts exist as crystalline solids with distinct physical and chemical properties.
Electrical Neutrality: The total positive and negative charges balance, so the compound has no net charge.
Sodium Fluoride (NaF): Added to toothpaste and drinking water to protect teeth.
Acids, bases, and salts interact in various reactions, with neutralisation being the most common. In a neutralisation reaction, an acid and a base react to form a salt and water. For example, HCl (hydrochloric acid) reacts with NaOH (sodium hydroxide) to produce NaCl (sodium chloride) and H₂O (water). Acids also react with metal carbonates to produce a salt, water, and carbon dioxide.
An example is the reaction between CaCO₃ (calcium carbonate) and HCl, forming CaCl₂ (calcium chloride), H₂O, and CO₂. Salts can further react with stronger acids or bases, or with each other in a double displacement reaction, often forming new salts or precipitates, depending on the solubility and strength of the reactants.
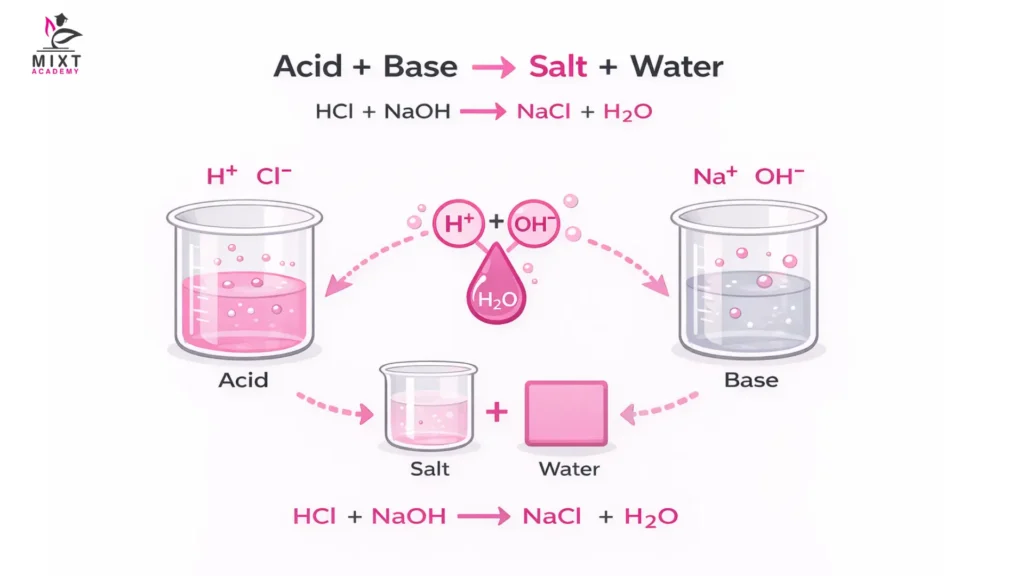
General Form: Acid + Base → Salt + Water
A salt can react with a stronger acid or base, especially if it was formed from a weaker acid or base. The reaction can produce a different salt or regenerate the original acid or base.
Salts can also react with other salts, particularly in solution, to form new salts. A common example of this occurs when a precipitate (an insoluble solid) is formed.
Salts are characterised by the combination of the acid and base used in their formation. Here are some examples of how salts are formed:
Mixt Academy certified chemistry tutors simplify salt formation using easy rules and guided practice, helping students identify salt types quickly and confidently.
Unlike acids and bases, which affect litmus paper (acids turn blue litmus paper red, and bases turn red litmus paper blue), salt solutions generally do not cause a colour change in litmus paper. This is because salts are typically neutral and do not significantly alter the hydrogen ion concentration.
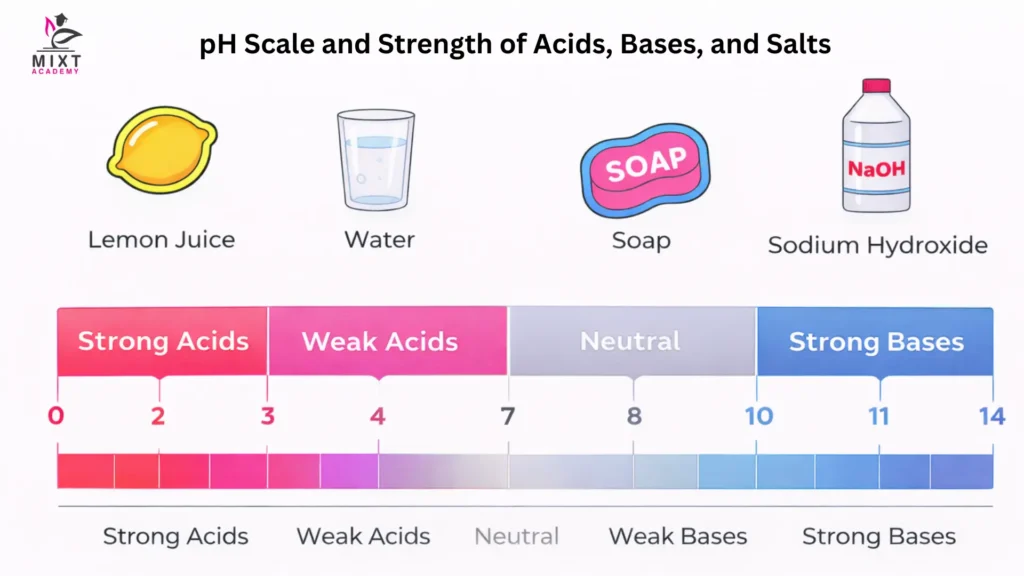
The pH scale is used to measure the acidity or basicity of a substance. It ranges from 0 to 14:
The lower the pH, the stronger the acid. For example:
Similarly, the higher the pH, the stronger the base. For example:
Each change of one unit on the pH scale represents a tenfold change in acidity or alkalinity, making it a logarithmic scale.
Substance | Key Properties | Example |
Acids | Sour taste, pH < 7, turns blue litmus red, releases H⁺ ions | Lemon juice, HCl |
Bases | Bitter, slippery, pH > 7, turns red litmus blue, releases OH⁻ ions | NaOH, soap |
Salts | Usually neutral, crystalline solids, formed by neutralisation | NaCl, CuSO₄ |
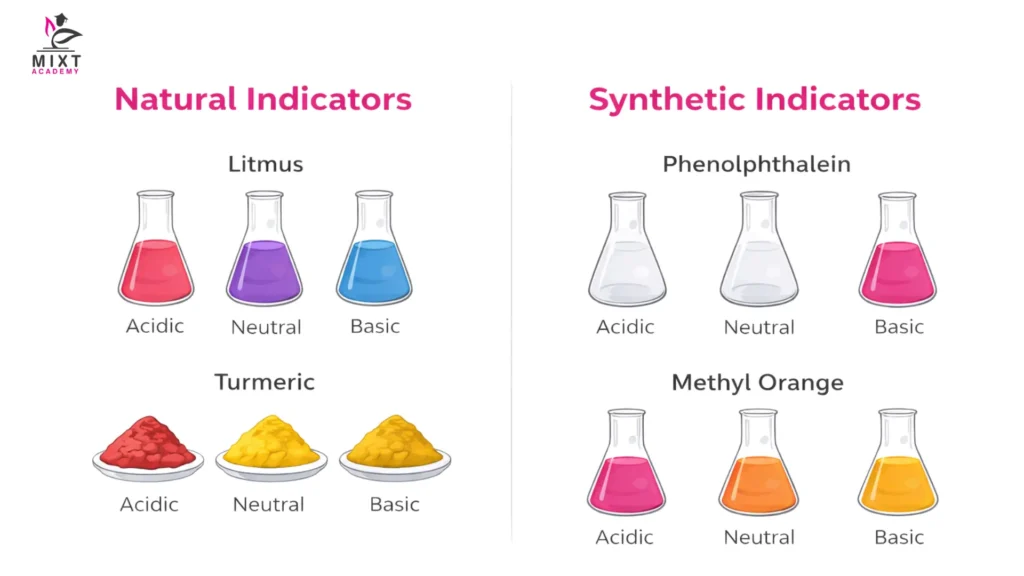
Indicators are substances that help determine whether a solution is acidic, basic, or neutral by changing colour. These tools are vital in chemistry, allowing for the identification of a substance’s pH. There are two primary types of indicators: natural and synthetic.
Indicators are chemical substances that change their colour based on the pH level of a solution. This colour shift occurs as the indicator reacts with hydrogen ions (H⁺) in acidic solutions or hydroxide ions (OH⁻) in basic solutions, providing a visual representation of whether the solution is acidic, neutral, or alkaline.
Synthetic indicators are artificial substances, usually made from complex organic compounds, that change colour in response to specific chemical conditions, typically at certain pH levels. These are commonly used in laboratory experiments and titrations for accurate pH measurement.
Type of Indicator | Definition | Characteristics | Examples |
Natural Indicators | Sourced from nature, such as plants and lichens | Often less precise with gradual colour changes; commonly used in simple tests | Litmus: Red in acid, blue in base Turmeric: Red in alkaline solutions |
Synthetic Indicators | Man-made substances created in laboratories | Provide sharp, distinct colour changes; ideal for precise chemical reactions | Phenolphthalein: Colourless in acid, pink in base Methyl Orange: Red in acid, yellow in base, orange in neutral |
This section is designed to help IGCSE and Class 10 students test their understanding of Acids, Bases, and Salts and prepare effectively for exams. Practising MCQs and structured questions improves accuracy, speed, and confidence for both school tests and board exams.
Answers: C
2. A substance that turns red litmus paper blue is most likely a:
A. Salt
B. Acid
C. Base
D. Neutral solution
Answers: 2–C
3.Which pair will undergo a neutralisation reaction?
A. HCl and NaCl
B. NaOH and HCl
C. CuSO₄ and H₂O
D. CaCO₃ and NaCl
Answers: 3–B
4.When an acid reacts with a metal carbonate, the products are:
A. Salt and water
B. Salt and hydrogen
C. Salt, water, and carbon dioxide
D. Water and carbon dioxide
Answers: 4–C
5.Which of the following is a weak acid?
A. Hydrochloric acid
B. Sulfuric acid
C. Nitric acid
D. Acetic acid
Answers: 5–D
Understanding Acids, Bases and Salts is important for mastering core chemistry concepts in IGCSE and Class 10. These substances explain everyday phenomena, from digestion and cleaning to food preservation and medicine. By learning their properties, reactions, and formation, along with the pH scale and indicators, students can confidently identify acidic, basic, and neutral substances.
Neutralisation reactions show how acids and bases interact to form useful salts, linking theory to real-life applications. With expert guidance and structured practice on the Mixt Academy online tutoring platform, students can improve these concepts through MCQs and exam-style questions. Mastering this topic not only improves academic performance but also deepens understanding of the chemical processes shaping the world around you.
Adding acid to water prevents splashing and excessive heat buildup. When water is added to concentrated acid, it can boil rapidly and cause dangerous spills. The correct method ensures safer dilution in laboratories.
Absolutely! These concepts are essential in medicine, agriculture, food processing, and environmental science. With Mixt Academy’s guided learning approach, students build knowledge that extends beyond exams into real-life understanding.
Not all salts are neutral. Salts formed from a strong acid and a weak base (e.g., ammonium chloride) are acidic, while those from a weak acid and a strong base (e.g., sodium acetate) are basic in nature.
Acids contribute to acid rain, which damages soil, crops, and buildings. Bases are used to neutralise acidic soils and industrial waste, helping protect ecosystems and maintain balance.
Mixt Academy provides structured online tutoring, interactive lessons, and exam-focused practice that help students clearly understand reactions, pH calculations, and real-world applications.

Mixt Academy is a global online tutoring platform that connects students with expert IGCSE, GCSE, and A-Level tutors for one-to-one learning. With flexible scheduling, personalized lesson plans, and experienced teachers from top curricula, Mixt Academy helps students strengthen concepts, improve exam skills, and achieve higher grades with confidence.
IGCSE Chemistry Papers: Common Mistakes & Exam Tips This IGCSE…
IGCSE Extended vs Core Tiers: How to Pick the Right…
Last Month Before IGCSE Exams: A Complete Study Plan Are…
Understanding GCSE Grade Boundaries and How to Prepare? Grade boundaries…
Differences GCSE English Language vs English Literature GCSE English is…
What is GCSE? A Guide for International Students & Parents…
Top Benefits of Completing AS and A Levels Privately Explore…
IB Math: Strategies for Achieving a Level 7 Learn expert…
How to Use OCR Past Papers for Effective Revision? Past…
Complete Guide to the AQA GCSE Chemistry Specification Understanding the…
Everything Students and Parents Must Know About AQA Exams Choosing…
AQA vs Cambridge: Comparing UK and International Exam Boards When…
Hire an Expert Tutor from Just 15$/hr