Before you study the isomers of butane, you need a clear grip on how carbon atoms bond and why structure matters more than you might expect. Many students jump straight to memorising structures. That creates confusion later. Let’s slow down and build the idea properly so the topic feels simple, not forced.
Organic chemistry starts with carbon behaviour. Carbon always forms 4 bonds. That single rule explains most structures you see later. Hydrocarbons are compounds made only of carbon and hydrogen. Among them, alkanes are the simplest group. Key points you should already understand:
For Any Alkane: CnH₂n+₂
Using this formula helps you check whether a structure is valid.
Example:
Many students assume one formula means one compound. That idea breaks here. Carbon atoms can link in more than one way while keeping the same molecular formula. When that happens, different compounds exist. These compounds are called structural isomers of butane because their bonding arrangement changes, not their formula.
A molecular formula shows only the number and types of atoms (e.g., ), which can represent different molecules like glucose and fructose that behave differently because their atoms are arranged differently.
A structural formula shows how atoms are bonded and arranged in space, which determines a molecule’s physical properties, chemical reactivity, and biological function.
One molecular formula does not always correspond to one compound because carbon atoms can bond in multiple ways, producing different compounds with identical formulas called isomers.
Butane Example: Butane () exists as two structural isomers with different bonding arrangements, resulting in different boiling points, reactivity, and physical behaviour.
A molecule’s function is directly determined by its structure, and even small changes in bonding or spatial orientation can significantly affect how it interacts with other substances.
An isomer is not created by bending or rotating bonds. Rotation does not change identity. An isomer appears only when:
Ask Yourself! Can I draw the carbon chain differently without breaking bonds? If yes, you may have an isomer.
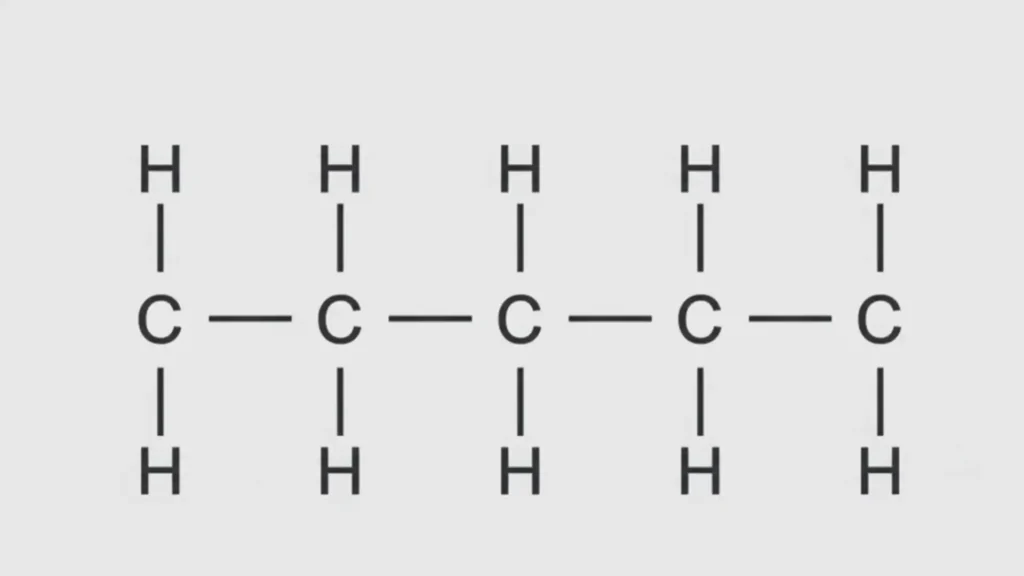
Butane contains four carbon atoms and ten hydrogen atoms. The carbons can be arranged in two distinct ways.
In one form, all four carbons connect in a continuous chain.
Structural Formula: CH₃–CH₂–CH₂–CH₃
This compound is called n-butane. The “n” stands for normal or straight chain.
Now rearrange the same four carbons.
Structural Formula: CH₃–CH(CH₃)–CH₃
This compound is known as isobutane, or by its systematic name, 2-methylpropane. Here lies the core n-butane and isobutane difference. The atoms stay the same. Their positions do not.
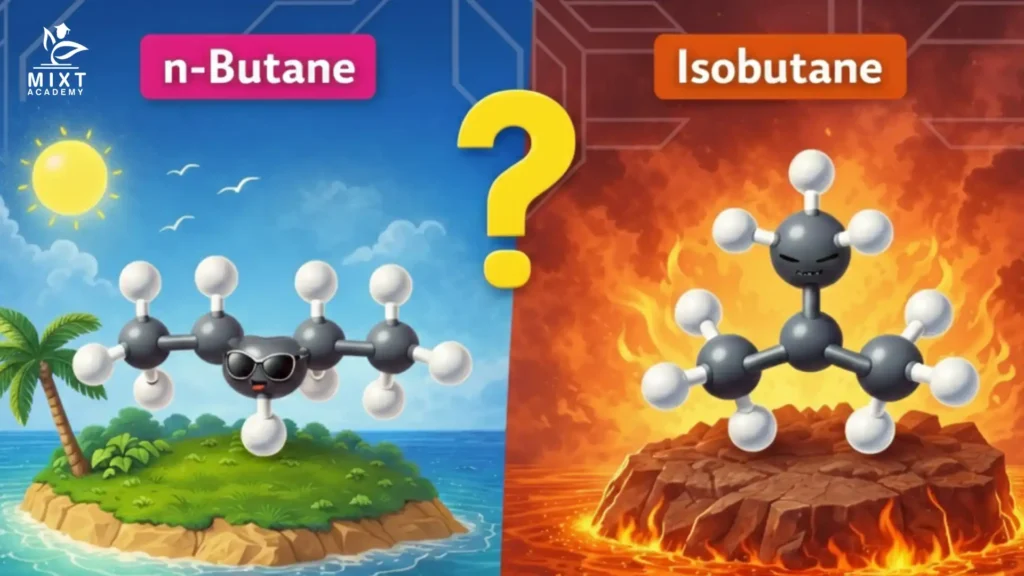
Structure affects physical behaviour. Key differences you should remember:
Measured Boiling Points:
Same formula with different behaviour.
You may see butane drawn in bent shapes. That does not mean new compounds exist. Rotation around single bonds only changes shape, not identity.
Tip For Exams:
Once you understand butane, larger alkanes feel easier.
The count rises as carbon numbers increase. This idea also prepares you to understand alcohol isomerism.
Example: Isomers of Butan-1-ol differ because the OH group attaches at different carbon positions, not because the carbon count changes.
Property | n-Butane | Isobutane |
Structure | Straight chain | Branched |
Carbon backbone | 4 continuous | 3 + branch |
Boiling point | Higher | Lower |
Shape | Less compact | Compact |
This topic trains you to:
Once you master this, topics like naming rules and reaction trends become easier.
Contact Mixt Academy for qualified online tutoring assistance according to your schedule.
Core Definition (Write This in Exams)
Butane is an alkane with the molecular formula C₄H₁₀. It shows structural isomerism because the four carbon atoms can be arranged in more than one way while keeping the same molecular formula.
Before answering any question on butane isomers, you should recall:
Structural isomers differ in carbon arrangement, not formula
These points are often asked indirectly.
Structural isomerism occurs when compounds have:
Butane shows chain isomerism, a type of structural isomerism. This is why it falls under structural isomers of butane.
Butane has only two structural isomers:
No other structural arrangement is possible for C₄H₁₀.
Labelled Structural Formula Diagrams
Condensed formula: CH₃–CH₂–CH₂–CH₃
Key Points:
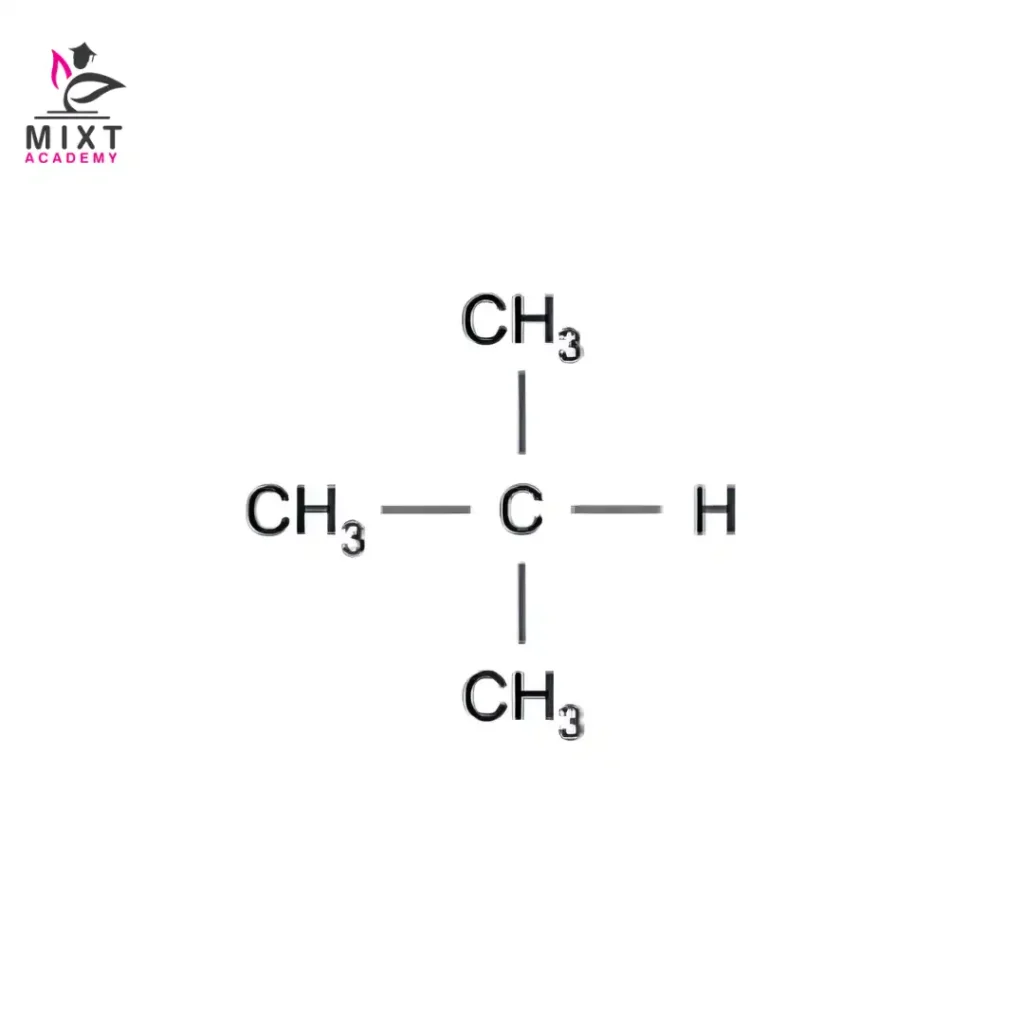
Key Points:
This structural change explains the n-butane and isobutane difference.
Reason: Branching reduces surface contact, lowering the boiling point.
Property | n-Butane | Isobutane |
Structure | Straight chain | Branched |
Type | Normal alkane | Branched alkane |
Boiling point | −0.5°C | −11.7°C |
Surface area | Larger | Smaller |
Intermolecular forces | Stronger | Weaker |
Struggling to visualise structural isomers of butane or understand the n-butane and isobutane? Mixt Academy is an online tutoring platform that simplifies organic chemistry through concept-based teaching, exam-focused notes, and one-on-one guidance. Learn at your pace, clear doubts instantly, and build strong fundamentals that boost exam confidence.
Expert chemistry concepts like isomers of butane with Mixt Academy qualified tutors.
Understanding the isomers of butane helps you see why structure matters more than memorising formulas. Although n-butane and isobutane share the same molecular formula, their different carbon arrangements lead to clear differences in physical properties like boiling point.
This idea explains the core of structural isomers of butane and prepares you for harder topics such as isomer counting, IUPAC naming, and even isomers of Butan-1-ol. Once you learn the difference between n-butane and isobutane, organic chemistry becomes manageable rather than confusing. In exams, this topic tests understanding, not memory, making it a high-scoring and concept-building area.
Butane has 2 structural isomers.
Butane follows the alkane general formula CₙH₂ₙ₊₂. For butane, n = 4, so the molecular formula is C₄H₁₀. There is no single formula to calculate the number of isomers; isomers are found by arranging the carbon atoms in different structural patterns.
The two isomers of C₄H₁₀ are: n-Butane, a straight-chain structure, and Isobutane (2-methylpropane), a branched-chain structure
C₆H₁₄ (hexane) has 5 structural isomers:

Mixt Academy is a global online tutoring platform that connects students with expert IGCSE, GCSE, and A-Level tutors for one-to-one learning. With flexible scheduling, personalized lesson plans, and experienced teachers from top curricula, Mixt Academy helps students strengthen concepts, improve exam skills, and achieve higher grades with confidence.
IGCSE Chemistry Papers: Common Mistakes & Exam Tips This IGCSE…
IGCSE Extended vs Core Tiers: How to Pick the Right…
Last Month Before IGCSE Exams: A Complete Study Plan Are…
Understanding GCSE Grade Boundaries and How to Prepare? Grade boundaries…
Differences GCSE English Language vs English Literature GCSE English is…
What is GCSE? A Guide for International Students & Parents…
Top Benefits of Completing AS and A Levels Privately Explore…
IB Math: Strategies for Achieving a Level 7 Learn expert…
How to Use OCR Past Papers for Effective Revision? Past…
Complete Guide to the AQA GCSE Chemistry Specification Understanding the…
Everything Students and Parents Must Know About AQA Exams Choosing…
AQA vs Cambridge: Comparing UK and International Exam Boards When…
Hire an Expert Tutor from Just 15$/hr